Pfizer’s COVID-19 Vaccine Receives Full FDA Approval
Amy Barczy
| 3 min read
Amy Barczy is a former brand journalist who authored...
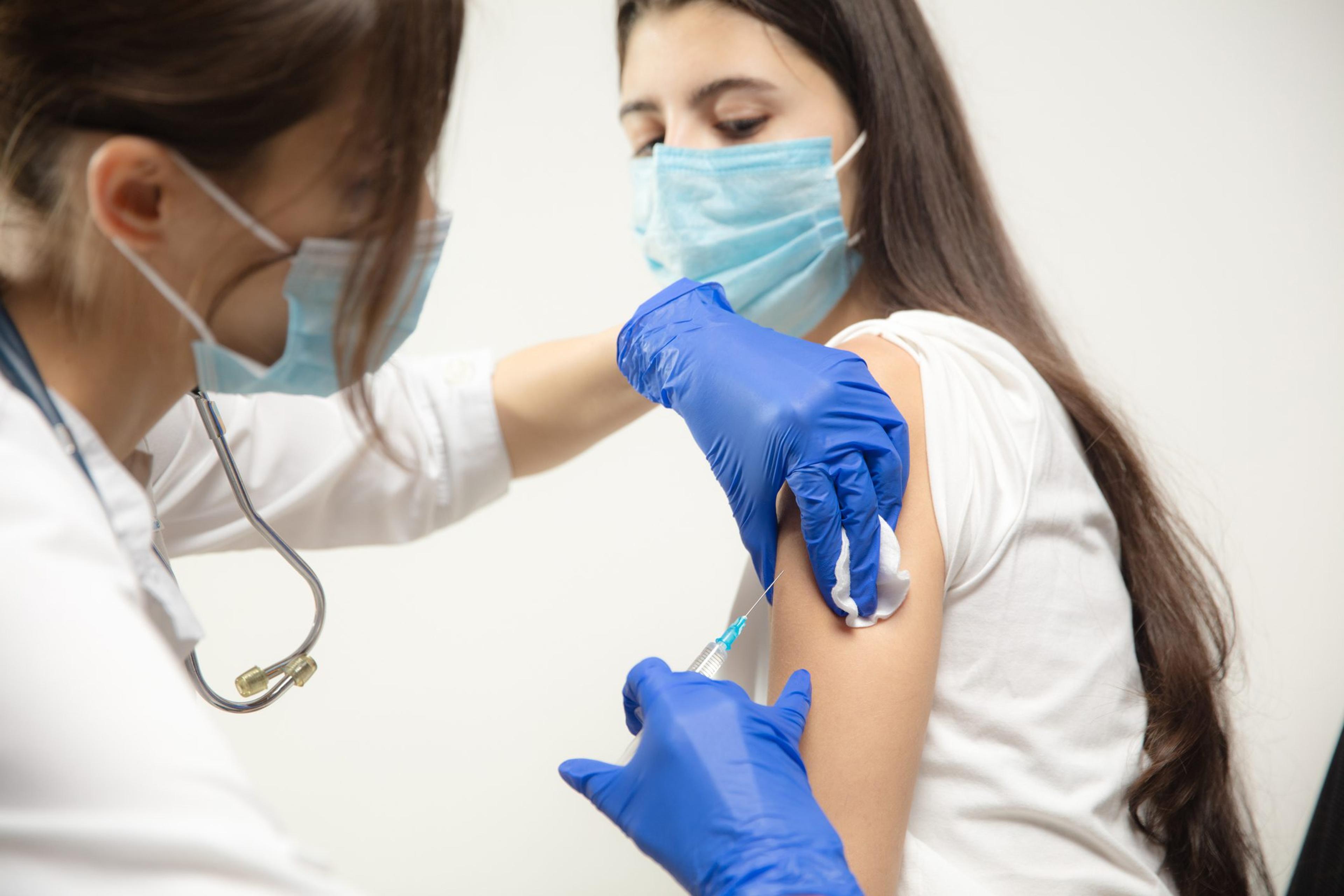
The U.S. Food and Drug Administration has granted its full approval to the Pfizer-BioNTech COVID-19 vaccine as of Aug. 23 for individuals 16 years of age and older. This full approval further supports the continued use of the two-dose Pfizer vaccine in the effort to combat the coronavirus pandemic and to save lives. The vaccine also continues to be available under emergency use authorization for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals. The Pfizer vaccine is effective in preventing illness from COVID-19 and more serious outcomes, including hospitalization and death. It’s commercial name on the market will be Comirnaty. Blue Cross Blue Shield of Michigan and Blue Care Network will continue to cover the cost of COVID-19 vaccine administration for our commercial population. Medicare is covering vaccine cost for Medicare beneficiaries. Members on our commercial and Medicare plans do not have a cost share for the vaccine. The FDA’s full approval builds on the previous emergency use authorization that the agency granted the Pfizer vaccine in December 2020. In deciding to approve the Pfizer vaccine, the FDA reviewed safety and effectiveness data from an ongoing Phase 3 clinical trial that was first used to grant emergency use authorization with thousands of participants. Before granting full approval, the FDA also reviewed additional safety and effectiveness data from a longer period in a larger group of people, including:
- Effectiveness data from 20,000 individuals who received the vaccine and 20,000 individuals who received a placebo, all ages 16 years old and older
- Safety data from 22,000 individuals who received the vaccine and 22,000 individuals who received a placebo, all ages 16 years old and older
- Safety surveillance data on reports of myocarditis and pericarditis after receiving the vaccine
Here’s the status of the FDA’s authorization for the Pfizer vaccine for different groups following today’s full approval:
- Individuals age 16 years old and up: Pfizer vaccine has full FDA approval
- Individuals age 12 to 15 years old: Pfizer vaccine has FDA emergency use authorization
- Individuals who are immunocompromised who need third dose “booster” shots: Pfizer vaccine has FDA emergency use authorization
Pfizer is also seeking emergency use authorization for a third “booster” dose of the vaccine for the general public, that would be administered 8 months after the initial vaccination round. The COVID-19 vaccines made by Moderna and Johnson and Johnson have emergency use authorization from the FDA for use in the U.S. in individuals age 18 years old and up. Both manufacturers plan to seek full FDA approval for their COVID vaccines in 2021. COVID-19 vaccines are widely available. Getting vaccinated is the best way to stop the spread of the coronavirus and to protect you and your family from serious illness. Blue Cross strongly encourages everyone who is eligible to be fully vaccinated for COVID-19.
More from MIBluesPerspectives:
- COVID Vaccine Updates: Get the Facts
- Why There are Still COVID-19 Hot Spots Across the U.S.
- Teens Can Get Their COVID Vaccines with Other Shots
Photo credit: Getty Images